Abstract
Background: Broadly, applicable strategies for liquid biopsies to diagnose and monitor lymphomas could transform patient management and potentially lead to novel therapeutic targets. MicroRNAs (miRNAs) are small non-coding RNAs that regulate post-transcriptional gene expression, contribute to various facets of cancer pathogenesis, and may potentially serve as novel therapeutic targets. We recently identified a key circulating serum miRNA signature (Beheshti et al Plos One 2017) in an in vivo spontaneous DLBCL model (ie, Smurf2T/T; Ramkumar et al. Nat Comm 2013) that was detectable many months prior to the formation of visible tumor. This signature consisted of 10 unique miRNAs (miR-15a, let-7c, let-7b, miR-27a, miR10b, miR-18a, miR-497, miR130a, miR24, and miR155) that each were present/overlapping in 2 lymphoid related organs (i.e., spleen and bone marrow) in addition to the serum in the Smurf2T/Tmodel. We hypothesized that this circulating miRNA signature would be similarly detected and elevated above non-tumor controls in serum of mice with DLBCL patient-derived xenografts (PDX) as well as serum from patients with DLBCL and that these miRNAs may be leveraged as an innovative therapeutic platform.
Methods: We utilized multiple MYC- rearranged and -unrearranged DLBCL PDX models (Townsend et al. Cancer Cell 2016) from tumors of patients with DLBCL. Following tumor transplantation, 200μL of serum was drawn from the PDX mice to analyze circulating miRNA. Serum from age-matched NSG mice without xenografts served as controls. We also analyzed circulating miRNAs in PDX models of peripheral T-cell lymphoma (PTCL) and several indolent B-cell lymphomas. Furthermore, we collected 2 mL of serum from 81 patients with DLBCL in the following conditions: untreated (active disease) (n=8); relapsed/refractory (with active disease) (n=11); long-term remission (24+ months) (n=25); and healthy non-tumor bearing individuals for control (n=8). Using Droplet Digital PCR (ddPCR), we quantified the 10 circulated miRNAs in the serum for all PDX and primary human samples. In addition, we targeted the top 4 predicted miRNAs from the signature utilizing novel self-deliverable antagomirs (AUM Biotech) in DLBCL cell lines (SUDHL2, SUDHL4, and SUDHL10) and performed in vitro assays to test cell viability after single antagomir treatment.
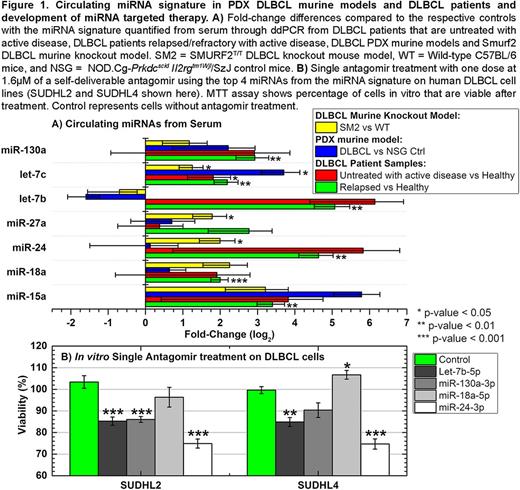
Results: Comparisons across models identified significant differences in serum miRNA expression between PTCL versus DLBCL PDXs and between indolent versus aggressive B-cell lymphomas ( P <0.05), but not between DLBCLs that had MYC alterations versus those lacking MYC alterations (i.e., molecular single translocation or double hits; or protein double expressers of Myc and Bcl2 protein). Examining quantitative ddPCR from serum obtained in the DLBCL PDX models after human tumor implantation, we identified that the miRNA signature was present and highly elevated in DLBCL PDX mice with prominent alterations confirmed in 7 of the 10 miRNAs (Fig. 1A). Furthermore, we identified significantly increased circulating levels of the miRNA signature from the serum of untreated and relapsed/refractory DLBCL patients with similar patterns of change as seen in the PDX models (Fig. 1A) that were independent of cell of origin (COO). In terms of leveraging these miRNAs as a potential therapeutic platform, we targeted the top 4 miRNAs from the aforementioned signature with antagomirs in several DLBCL cell lines (i.e., germinal center and non-germinal center) with one acute dose at 1.6µM for each single self-deliverable antagomir treatment. Four days following this treatment, there was a significant decrease ( P <0.01) in cell viability noted for 3 of the 4 miRNAs examined (Fig. 1B).
Conclusions: Collectively, we show here that circulating miRNAs established using a murine knockout model of DLBCL have broader application to serum from PDX models and patients with human lymphoma. The circulating miRNA alterations in DLBCL patients appeared to be independent of both MYC alterations and COO, suggesting a broadly applicable approach. Finally, the ease of detection and quantification in as little as 200μL of serum makes this approach an attractive strategy for prospective validation in larger patient cohorts.
Evens: Affimed: Consultancy; AbbVie: Consultancy; Seattle Genetics: Consultancy; Amgen: Consultancy; Millennium: Consultancy; Novartis: Consultancy; Pharmacyclics: Consultancy; • Spectrum Pharmaceuticals: Consultancy; Celgene: Consultancy; Kite Pharma: Consultancy; Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal